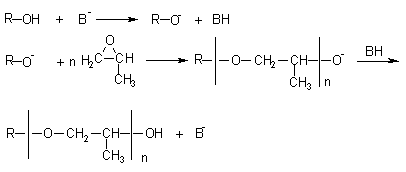
A great polyol variety
is used in PU's. Among the most widely employed are polyether polyols, which
are obtained by the polymerization of propylene, ethylene and butylene oxides.
Most commonly used are poly (propylene oxide) glycol and copolymers of (propylene/ethylene
oxides) glycols (PPG's) (Table 1.5). Other polyether polyols, such as poly (tetramethylene
oxide) glycol, are used in PU fibers and high performance elastomers, while
polymeric polyols are used in high resilience flexible foams. Besides, one can
cite the polyester polyols (Table 1.6), used in high performance applications,
castor oil, hydroxyl-terminated polybutadiene (HTPB), etc. Usually, polyols
having molecular weight between 1,000 and 6,000 and functionality between 1.8
and 3.0 are used in flexible foams and elastomers. Short chain (250
1.3.1 - Polypropylene glycols (PPG's)
PPG's are obtained through anionic polymerization of propylene oxide (PO) and propylene and ethylene oxides (EO) copolymerization. The first stage of the PPG polymerization process is the reaction of an alcohol with a strong base (usually potassium hydroxide), forming the corresponding alcoholate. Figure 1.22 shows the mechanism of secondary hydroxyl formation resulting from the nucleophilic attack to the less hindered PO carbon atom.
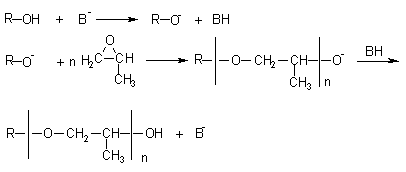
Figure 1.22 - PPG preparation
PPG's narrow molecular weight distribution is due to the anionic polymerization process. The polyether functionality corresponds to the functionality of hydroxyl or amine group-containing compounds used as initiators (Tables 1.5 and 1.6). The PPG's viscosity is between 100 and 1,000 cP @ 25°C. PPG's diols of MW between 400 and 4,000 and hydroxyl number from 265 to 28 mg of KOH/g are used in CASE products, and the trifunctional ones, of molecular weight between 3,000 and 6,000, hydroxyl number from 56 to 28 mg of KOH/g are used in flexible foams, those of higher MW being used in high resilience foams (HR). High functionality polyols, of MW lower than 1,000, hydroxyl number between 300 to 800 and high viscosity (in some cases until 17,000 cP @ 25°C) yield highly crosslinked polyurethanes and are used in rigid foams. A few examples of polyether polyols based on propylene and ethylene oxides, used in different applications, are shown in Table 1.5.
Table 1.5 - Typical properties of polyether polyols|
Applications |
CASE1 |
Flexible Foams |
Rigid Foams |
|||
|
Conventional |
Conventional |
HR |
||||
|
Polyol composition |
propylene glycol + propylene oxide |
glicerine + propylene and ethylene oxides |
amine + propylene oxide |
trimethylolpropane + propylene and ethylene oxides |
trimethylolpropane + propylene oxide |
sucrose + propylene oxide |
|
Average MW |
2000 ± 100 |
3000 ± 200 |
3750 ± 200 |
4800 ± 300 |
440 ± 35 |
860 ± 60 |
|
OH number (mg KOH/g)
|
56 ± 3
|
56 ± 3
|
60 ± 3
|
35 ± 2
|
380 ±
25
|
380 ± 25
|
|
OH content (meq/g) |
1.0 |
1.0 |
1.1 |
0.6 |
6.8 |
6.9 |
|
Average functionality2 |
2.0 |
3.0 |
4.0 |
3.0 |
3.0 |
5.8 |
|
Insaturation (meq/g) |
< 0.04 |
0.04 |
< 0.04 |
< 0.05 |
< 0.005 |
< 0.005 |
|
Viscosity at 25oC (mPa.s)
|
250 – 350 |
450 – 550 |
580 – 720 |
750 – 900 |
600 - 700 |
11000 – 15000 |
|
Pour point (oC)
|
- 36 |
- 31 |
- 35 |
- 38 |
- 22 |
- 2 |
|
pH |
6.5 – 8.0 |
6.5 – 8.0 |
8.6 – 9.6 |
6.5 – 8.0 |
6.0 – 7.5 |
6.5 – 8.0 |
|
Density, 25oC
(g/cm)
|
1.00 |
1.01 |
1.00 |
1.02 |
1.03 |
1.1 |
Usually, diols such as propylene glycol are used as initiators for the production of polyether diols; triols such as glycerin and trimethylol propane to obtain polyether triols, and products of higher functionality such as sorbitol and sucrose in the production of high functionality polyether polyols (Table 1.6).
Table 1.6 - Common initiators for polyether polyols|
Initiators |
Structure |
Functionality |
|
Water |
HOH |
2 |
|
Ethylene glycol |
HOCH2CH2OH |
2 |
|
1,2-Propylene glycol |
HOCH2CH(CH3)OH |
2 |
|
Glicerine |
|
3 |
|
Trimethylolpropane |
|
3 |
|
Triethanol amine |
N-(-CH2-CH2OH)3 |
3 |
|
Pentaerythritol |
C-(-CH2OH)4 |
4 |
|
Ethylene diamine |
H2NCH2CH2NH2 |
4 |
|
2,4-toluene diamine or (2,6-toluene diamine) |
|
4 |
|
4’.4’-diphenyl methane diamine |
|
4 |
|
Diethylene triamine |
H2NCH2CH2NHCH2CH2NH2 |
5 |
|
Sorbitol |
|
6 |
|
Sucrose |
|
8 |
Primary amines can also be used as initiators to obtain polyether polyols. Due to the stronger nucleophilic character of the amines as compared to hydroxylated compounds, the use of KOH as catalyst may be dispensed with. Ethylene and toluene diamines are initiators for obtaining tetra functional polyether polyols. The higher basicity of the resulting polyols having tertiary amine groups makes them more reactive towards isocyanates.
1.3.1.1 - Copolymers with ethylene oxide
Reactive PPG's are used in cold molded PU foam systems, and their reactivity depends on the degree of primary hydroxyl groups (Figure 1.23). PPG's produced only with PO possess less reactive secondary hydroxyl groups. For obtaining more reactive primary hydroxyls, reaction with EO is carried out in the final stage of the polymerization. Usually the EO block is less than 20% of the polymer chain and it improves the polyol solubility in water. PO/EO copolymers increase the water solubility and decrease the heterogeneous phases, where the reaction of water with isocyanate occurs and forms rigid polyurea macrophases, resulting in softer flexible foams (Chapter 3).
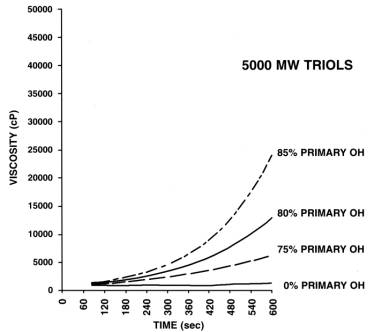
1.3.1.2 - Low monol degree PPG's
The conventional propylene oxide based polyether polyols (PPG) are produced commercially through the base catalyzed, using an alkali metal salt such potassium hydroxide (KOH), propoxylation of so-called starters, which have two or more hydroxyl groups. Typically, propylene glycol is used to produce diols and glycerin to produce triols. However, their functionality is slightly lower than that of the initiator compound, this sometimes being due to small amounts of remaining water in reagents. It is well known that base catalyzes not only the addition of propylene oxide to the growing polyol molecule, but also a side reaction in which propylene oxide isomerizes to allyl alcohol (Figure 1.24).
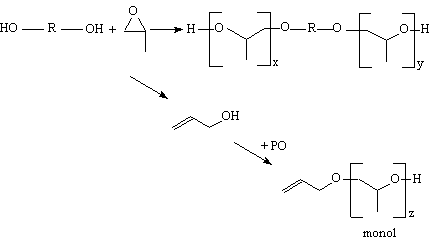
Figure 1.24 - PPG monol formation
Allyl alcohol acts
as a monofunctional starter resulting in propoxylated allyl alcohol referred
to as monol. These monofunctional products act as chain terminators during the
PU formation, resulting in a decrease in mechanical properties. As PPG monol
is double bond- terminated, the monol degree can be quantified by the insaturation
(in meq/g). The PPG's insaturation level or monol content increases with the
molecular weight (Table 1.7). For example, a 2000 MW conventional PPG diol has
a monol content of about 0,03 meq/g, which correspond to a functionality of
1,92, whereas, a 4000 MW PPG diol has a monol content of about 0.085 meq/g and
functionality of only 1.7. Syntheses of low insaturation degree (<0.02 meq/g)
polyether polyols have been carried out by using double metal cyanide (DMC)
catalysts based on Zn3[Co(CN)6]2
(zinc hexacyanocobaltate). The new generation of ultra low monol
content polyols has a typical monol (insaturation) content of 0.005 meq/g or
less, which correspond to a functionality of 1.98 for a 4000 MW diol.
|
Molecular Weight |
OH number
(mg de KOH/g) |
Viscosity at (25oC)
|
Insaturation (meq/g) |
Monol % (mol)
|
Average Functionality* |
|
|
Conventional |
Low monol
|
|||||
|
1000 |
111 |
145 |
0.01 |
1 |
1.99 |
|
|
1000 |
111 |
145 |
0.005 |
0,5 |
1.995 |
|
|
2000 |
56 |
335 |
0.03 |
6 |
1.94 |
|
|
2000 |
56 |
335 |
0.005 |
1 |
1.99 |
|
|
3000 |
37 |
570 |
0.05 |
14 |
1.86 |
|
|
3000 |
37 |
580 |
0.004 |
1 |
1.99 |
|
|
4000 |
28 |
980 |
0.09 |
31 |
1.69 |
|
|
4200 |
28 |
860 |
0.005 |
2 |
1.98 |
|
|
8000 |
14 |
- |
- |
- |
||
|
8200 |
14 |
3000 |
0.05 |
4 |
1.96 |
|
Polyether polyols can be "filled" with grafted organic polymers. The successful preparation of stable, colloidal dispersions requires that the particles have some type of steric stabilization to prevent flocculation of the suspension. These white and viscous modified polyol dispersions are useful in making high hardness foams. Polymeric polyols are usually formed trough a free radical or step addition process by in situ monomer grafting to PPG. There are two types of modified polyether polyols.
Copolymer Polyols - Copolymer polyols are obtained through free radical grafting of styrene and acrylonitrile (SAN copolymer) to PPG. The product contains a mixture of PPG, SAN copolymer and graft copolymer polyols that act as a stabilizer (Figure 1.25).
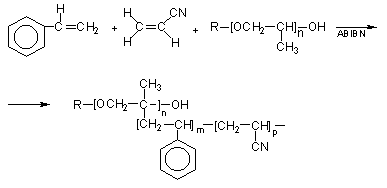
Figure 1.25 - Copolymer polyol reactions
The first commercial copolymer polyols were based on acrylonitrile as the sole monomer. This dispersion contained 20% poly(acrylonitrile) and had viscosities of 3,000-5,000 mPa.s. These products were used for the production of cold molded, high resilience flexible foams (HR), with improved hardness, strength, foam processing, and cell opening. As the 100% acrylonitrile copolymer polyols cause discoloration problems in slabstock flexible foams, styrene/acrylonitrile (SAN) copolymer polyols were developed. These copolymer polyols require the use of a more efficient stabilizer molecule to form the grafted portion of the polymer. This is because the styrene and acrylonitrile copolymerization is quite favorable, and the tendency for free radicals to be generated on the polyol is reduced. The so-called macromer stabilizers are PPG's which have been functionalized with a vinyl moiety (for the PPG's reaction with maleic anhydride, ethylisocyanate methacrylate, methacryloyl chloride, etc), which will undergo copolymerization with SAN monomers (Figure 1.26).
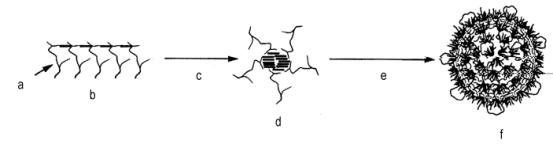
Polymerization starts with the addition of base polyol and macromer, followed by initiator and monomer feed. In the beginning the reaction mixture is homogeneous and the grafted polyol is formed. At some point (corresponding to 1-3% of total added monomer) the polymeric "comb" associates into a spherical structure, in which the insoluble copolymer is located in the center of the sphere, and the macromer chains are in the surface, where they can interact with the continuous polyol phase. Polymerization continues inside the particles that grow from 0.01-0.05 to 0.3-0.5 microns, yielding a narrow distribution and monodisperse size particles. In molded and slabstock HR flexible foams copolymer polyols are used with 25-40% solids and viscosity 2,500-7,000 mPa.s, and for conventional flexible foams, with 40-43% solids and 4,000-6,000 mPa.s.
PHD Polyol (urea/urethane polyol) - PHD polyols (polyurea modified) are dispersions of polyurea particles, formed by the reaction between TDI and diamines (hydrazine, ethylene diamine, etc) in a conventional polyol. In the reaction of diamines and isocyanates, the low molecular weight polyurea formed separates from the continuous phase. Since the reaction of a polyol and diisocyanate is slower than that of a diamine and diisocyanate, their tendency to form small particles is less probable than that of the SAN copolymer polyols. This leads to lower incorporation of polyol grafts and broader distribution of particle sizes. The diamine quickly reacts with an excess diisocyanate and the formed polyurea- modified isocyanate reacts with the polyol, forming poly(urea/urethane) that acts as stabilizer for the polyurea dispersion in the PHD polyol (Figure 1.27). These polyureas, present in 20-30%, react with isocyanate during foam manufacture, eventually increasing the cross-linking degree.
|
|
Figure 1.27 - Formation of PHD polyol