Chapter 2 - Additives
Several reagents are used in PU production, such as: isocyanates (Chapter 1.2), polyols (Chapter 1.3), polyamines (Chapter 1.4), chain extenders and crosslinkers (Chapter 1.5). Besides these, a great variety of chemical products can be added to control or modify the PU's formation as well their final properties. These additives include: catalysts, blowing agents (Chapter 2.3); surfactants (Chapter 2.4); fillers (Chapter 2.5), antiaging agents (Chapter 2.6), colorants (Chapter 2.7), flame retardants (Chapter 2.8), mold release agents (Chapter 2.9), lubricants, adhesion promoters, solvents, plasticizers, rheology promoters, moisture scavengers, etc.
In terms of industrial productivity, in the absence of catalysts the isocyanate group reacts rather slowly with alcohols, water and itself. The catalyst choice for PU's manufacture is usually directed for obtaining an appropriate profile among the several reactions that can occur during PU production processes. Catalysts are used for producing cellular PU's (flexible, semi-rigid and rigid foams, microcellular elastomers) as well as solid PU's (elastomers, coatings, sealants, adhesives, etc). Different catalyst types are used for the reaction of isocyanate with water and polyols, the catalysts usually being aliphatic or aromatic tertiary amines, or organometallic compounds. Normally, tertiary amines are used for catalyzing the polyaddition reaction of isocyanate with a polyol-forming PU, as well as in the blowing reaction of isocyanate with water, forming polyurea and carbonic gas as blowing agent. Organometallic catalysts are mainly used in the catalysis of the polymerization reaction of an isocyanate with a polyol. Carboxylic acid salts of alkaline metals, phenols and symmetrical triazines are used to catalyze the polymerization of isocyanate with isocyanurate formation.
Catalysts (Table 2.1) are used to promote selectivity when different chemical reactions occur at the same time, as is the case with PU's. PU's final properties depend on the amount of urethane, urea, allophanate, biuret, and isocyanurate bonds, along the polymer chain. On the other hand, these bonds depend on the type and concentration of the catalyst or its mixtures. This means that catalysts exert a considerable influence on PU structures and its end properties.
Table 2.1 - Catalysts used in PU|
Reaction |
Catalysts |
|
NCO/NCO - trimerization |
strong bases (potassium octoate), quaternary ammonium, phosphines |
|
NCO/NCO - dimerization |
phosphorous compounds |
|
NCO/NCO - polymerization
|
alkaline metal hydroxides |
|
NCO/OH |
tertiary amines, organometals, metallic soaps |
|
NCO/H2O |
tertiary amines |
|
NCO/NHCOOR (urethane) |
metallic soaps |
|
NCO/NHCONHR (urea) |
tin and zinc soaps |
The growing catalyst basicity promotes improved crosslink formation (alophanate and biuret). Generally, with tertiary amines the higher basicity increases the catalytic effect, except in the occurrence of steric hindrance. Triethylene diamine (TEDA) or 1,4-diazo (2,2,2)-bicyclo-octane (DABCO) have a stronger catalytic effect due to the absence of steric hindrance. It is important to emphasize that the catalyst specificity can vary according to the system used, hence the danger of trying to extract correlations from studies done in different systems. Basically the catalyst should be sufficiently nucleophilic to stabilize the isocyanate group by resonance, or to activate the active hydrogen atom-containing compound. According to the basic catalysis mechanism show in Figure 2.1, initially occurs the formation of a complex between the base and the isocyanate group, activating the NCO group and making easier the reaction with the non-paired electrons of the alcohol oxygen atom. Afterwards the complex is decomposed, forming PU and regenerating the base.
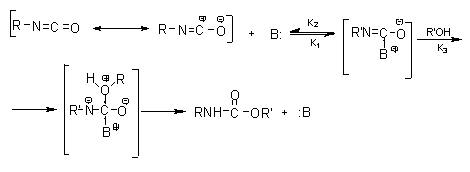
Figure 2.1 - Basic catalysis mechanism
Different catalysts (tertiary amines, alkaline compounds and organo metals) have different catalytic effects on the isocyanate group reactivity with active hydrogen atoms (Table 2.2) of urethane, urea, water and alcohol groups.
Table 2.2 - NCO group reactivity with active hydrogen-containing compounds| Hydrogen-containing compounds |
Non catalyzed reaction
|
Tertiary amines1
|
Alkaline compounds2
|
Organometals3
|
|
Urethane |
1 |
- |
strong |
- |
|
Urea |
100 |
- |
strong |
weak |
|
Water |
400 |
strong |
strong |
weak |
|
Alcohol |
400 |
strong |
strong |
very strong |
In Table 2.2, it may be seen that organometals have a very strong effect on PU formation, while the effect is weak on the blowing reaction, between isocyanate and water. Tertiary amines show a strong catalytic effect on PU formation and also on the blowing reaction, and both have a weaker effect on allophanate and biuret formation. This is very important in PU foam technology where organometals are commonly used to catalyze PU formation while tertiary amines are employed to catalyze the blowing reaction.