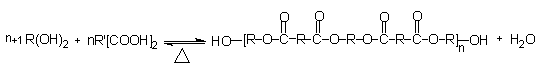
Polyester polyols were the first polyols used in the beginning of PU development, and are produced by polycondensation of a diacid with excess diol (Figure 1.28).
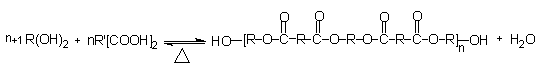
Figure 1.28 - Obtaining polyol polyester
Difunctional monomers are used to obtain a linear polymer; and monomers with functionality larger than two as trimethylol propane and glycerin create ramified chains. The most used acids are adipic and phthalics. Adipic acid based polyester polyols are used in applications where flexibility is wanted, as in flexible foams and elastomers. Phthalic acids (or phthalic anhydride) based polyols, have rigid chains and are used in rigid foams and in high performance coatings (Table 1.8).
Table 1.8 - Typical properties of polyesters polyols|
Application
|
Flexible
foam
|
Semi-rigid foam
|
Rigid foam
|
Shoe soles |
Elastomers |
Coatings |
|
|
soft
|
hard
|
||||||
|
Structure |
adipic acid, diethylene glycol, trimethylol propane |
adipic acid phthalic acid, 1,2-propylene glycol, glycerine |
adipic acid, phthalic acid, oleic acid, trimethylol propane |
adipic acid, ethylene glycol, diethylene glycol |
adipic acid, ethylene glycol, 1,4-butane diol |
adipic acid, diethylene glycol |
phthalic acid, maleic acid, trimethylol propane |
|
Average MW |
2400 |
1000 |
930 |
2000 |
2000 |
2750 |
2450 |
|
OH number (mgKOH/g) |
57 - 63 |
205 - 221 |
350 - 390 |
58-62
|
52 - 58 |
38 - 45 |
250 - 270 |
|
OH content (meq/g) |
1.1 |
3.8 |
6.6 |
1.1 |
1.0 |
0.7 |
4.6 |
|
Average functionality* |
2.7 |
3.8 |
6.2 |
2.1 |
2.0 |
2.0 |
11.3 |
|
Viscosity at 75°C (mPa.s) |
950 - 1100 |
570 - 750 |
1300 - 1550 |
500 – 700 |
500 - 600 |
700 - 800 |
17000 a 150oC |
|
Pour point (°C) |
-12 |
-12 |
7 |
17 to 56 |
49 to 52 |
-9 |
90 to 100 |
|
Acid number |
1.2 |
2.8 |
1.0 |
0.4 |
1.0 |
1.0 |
4.0 |
|
Density, 75°C (g/cm) |
1.15 |
1.15 |
1.1 |
1.15 |
1.17 |
1.12 |
1.24 |
In polyester polyols production process, the diol, triol, etc is first heated to a temperature of 60-90°C. Then the dicarboxilic acid is added and removal of the reaction water begins. For obtaining the targeted molecular weight the excess diol is calculated by means of Flory Equation. Diol can be lost during removal of the water form the condensation reaction and through side reactions (formation of ethers and aldehydes). The amount of diol lost is dependent upon the processing conditions and upon type of diol. The amount of diol lost must be empirically determined. Usually the reaction is completed at temperatures up to 200°C. Nitrogen, carbon dioxide, or vacuum is used to remove the water and to reach the wanted conversion of 99.9%, and the resulting polyester should have an acid number less than two. This conversion is necessary to minimize the presence of residual carboxylic end groups that can reduce the reactivity. The polyesters are composed of all possible oligomers raging from the monomers to high molecular weight species: the molecular weight distribution follows a Frory probability. The properties of the PU based polyester elastomers are governed mainly by the overall molecular weight of the polyester and only to a minor degree by the molecular weight distribution.
Acids, bases and compounds of the transition metals can catalyze the esterification reaction. The dicarboxylic acids also exert a limited catalytic effect. In practice catalysts are used reluctantly because they cannot be removed and can have an undesirable effect on the following PU reaction, since inorganic substances even in the smallest quantities favor or retard de PU processing reaction. The p-toluenesulphonic acid can be used as an accelerator and left in the polyester. In cases where small amounts of catalysts do not later cause problems, compounds of tin, antimony, titanium, lead and other metals, have proved especially effective. The amounts added lie in the ppm range. Solid impurities are removed by hot filtration of the finished polyester.
Usually aliphatic polyester polyols used in flexible polyurethanes are based on polyadipates diols such as ethylene glycol, diethylene glycol, propylene glycol, 1,4-butane diol, 1,6-hexane diol, etc. The growth of the diol chain results in greater PU flexibility and hydrolytic stability and reduction of polarity and glass transition temperature. Polyester polyols used in PU elastomers (Chapter 6), based on acid adipic and a glycol like ethylene glycol, 1,4-butane diol, 1,6-hexane diol, neopentyl glycol or mixtures of them (Table 1.9), are crystalline products with melting point between 50 and 60oC. The crystallinity can be reduced using mixed diols (as 1,4-butane diol and ethylene glycol) or mixed polyesters.
Lightly branched poly(diethyleneglycol adipates), which are used mainly to make flexible foams, and a wide range of adipates made with more than one aliphatic diol. These are used to make solid and microcellular elastomers, flexible coatings and adhesives. Relatively low cost polyester polyols, based on recovery materials are also available. Mixed adipic, glutaric and succinic acid polyesters are made using purified nylon waste acids (AGS acids). AGS acids are also hydrogenated to make a mixture of 1,4-butanediol, 1,5-pentanediol and 1,6-hexane diol, which is used to make polyadipates having a low melting point. Mixed polyadipates from hydrogenated AGS acids are used to make microcellular elastomers with good hydrolytic stability.
Table 1.9 - Polyester polyols of MW = 2000|
Structure |
Solidification point (°C) |
Viscosity at 75°C (mPa.s) |
|
adipic acid + ethylene glycol |
52 |
540 |
|
adipic acid + ethylene glycol + 1,4-butane diol |
17 |
625 |
|
adipic acid + 1,4-butane diol |
56 |
670 |
|
adipic acid + hexamethylene glycol + neopentyl glycol |
27 |
640 |
In comparison with PU based polyether polyols, the PU based polyesters are more resistant to oil, grease, solvents and oxidation. They possess better properties related to: tension and tear strength, flex fatigue, abrasion, adhesion and dimensional stability. On the other hand PU based esters are more sensitive to hydrolysis and microbiological attack. The high mechanical properties of PU based polyester can be explained by the greater compatibility between polar polyester flexible segments and polar rigid segments, causing a slower phase separation resulting in better distributed small crystalline rigid blocks (Chapter 1.7).
The hydrolysis stability of the ester linkage is inferior to that of the ether linkage in the polyethers, and residual esterification catalysts accelerate the hydrolysis. The hydrolysis resistance of the polyol polyester based PU increases with long chain glycols (1,6-hexane diol) or long chain diacids (dodecanoic acid), as a result of the largest hydrophobic portion and small amounts of ester groups. The hydrolysis stability can be improved with additives that react with carboxylic and alcoholic groups, formed during hydrolysis. These additives may be: oxazolines, epoxy compounds, aromatic polycarbodiimides and aliphatic monocarbodiimides. TPU's based polyester polyols are stabilized by addition of 1 to 2% in weight of aromatic hindered carbodiimides, that react with the acid generated by ester hydrolysis, which would act as catalyst of hydrolysis reactions (Figure 1.29).
![]()
Figure 1.29 - Reaction of carbodiimides with carboxyl
Polymeric polyester polyols are dispersions of vinyl polymers in polyadipate based polyol polyester stabilized by a dispersant. Polymeric polyester polyols, containing 10 to 20% of vinyl polymers are used in shoe soles and flexible PU foams with greater hydrolysis stability, higher hardness for same densities, more uniform cellular structures, and better dimensional stability.
1.3.2.1.1- Polycaprolactone polyols
Another process for production of aliphatics polyester polyols includes the ring opening polymerization of e-caprolactone with glycols (Chapter 1). Polycaprolactone diols are produced with diethylene glycol, 1,4 butane diol, neopentyl glycol or 1,6-hexane diol. The polycaprolactone triols use trimetilol propane or glycerin /ethylene glicol, and the tetrols are done with pentaerythritol. The polycaprolactone glycols are produced with MW from 400 to 4000, hydroxyl number from 560 to 28 mg KOH/g, and they have greater hydrolysis resistance and lower viscosity than the polyadipate glycols of same MW. They are used in production of high resistance PU, modification of resins, coatings, adhesives, shoe soles and orthopedic goods. Polycaprolactone and polyadipate copolymers diols are usually liquids of low viscosity at room temperature.
1.3.2.2 - Aromatic polyester polyols
Aromatic polyester polyols based on terephthalic or isophthalic acids are used in high performance hard coatings and adhesives, and in polyurethane (PUR) or polysocyanurate (PIR) rigid foams resistant to fire. In combustibility tests, the PIR and PUR foams based on aromatic polyester polyols form a charred backbone, and in many formulations reduces or eliminates the use of fire retardantes.
The polyterephthalate glycols are usually obtained by polymerization of dimethyl terephthalate (DMT) with ethylene glycol. Polyols with average equivalent weight of 181, functionality 2.3, hydroxyl number between 295 and 335 mg KOH/g, viscosities from 8,000 to 22,000 cP at 25°C, can be used in rigid foams and foundry systems. The ones with average equivalent weight of 238, functionality 2.0, hydroxyl number between 230 and 242 mg KOH/g, viscosity from 2,700 to 5,500 cP at 25°C, are used in PIR foams with minimum shrinkage and high weight retention. The ones with average equivalent weight 167, functionality 2.0, hydroxyl number between 315 and 350 mg KOH/g, viscosity of 1,300 to 3,000 cP at 25°C, are used in appliance thermal insulation and other low viscosity applications. Another polyterephthalates glycols obtaining process uses high molecular weight poly(ethylene terephthalate) (PET) scraps of polyester fibers or soft drinks bottles. The low molecular weight polyols are obtained by transesterification of milled PET residues with propylene glycol or mixture of ethylene/propylene glycols at 216°C for about 6 hours.
The polyisophthalates glycols are obtained by anhydride phthalic polymerization with glycols as diethylene glycol. The poly(diethylene isophthalate) glycol with average equivalent weight of 178 and 234, OH number of 230 to 330 mg KOH/g, viscosities from 2,000 to 4,500 cP at 25°C are used in PUR and PIR foams. The ones with equivalent weight of 288, OH number of 195 mg KOH/g, viscosity of 25,000 cP at 25°C can be used in resins and prepolymers for coatings, adhesives, sealants and elastomers, and also as additive in polyol polyether flexible foams to improve fire resistance and adhesion characteristics. The poly(neopentyl isophthalate) glycols with average equivalent weight of 510, OH number of 110 mg KOH/g are used in adhesives, coatings and elastomers with excellent hydrolysis resistance.
1.3.3 - Polytetramethylene ether glycols (PTMEG or PTHF)
The poly(oxytetramethylene) glycol or polytetramethylene ether glycol (PTMEG) are manufactured by the cationic polymerization of tetrahydrofuran (THF) (Figure 1.30). PTMEG's are linear chain polyols with reactive primary hydroxyls and functionality of 2.0. PTMEG's of molecular weights of 650, 1000 and 2000 (Table 1.10), are used in high performance PU and TPU's elastomers, coatings and elastomeric fibers.
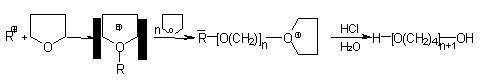
Figure 1.30 - Obtaining of PTMEG's
PTMEG's are solid, white, waxy at room temperature, soluble in alcohols, esters, ketones and aromatic and chlorinated hydrocarbons, and insoluble in aliphatic hydrocarbons and water. They have variable solubilities for glycols: poly(oxypropylene) glycols and 1,6 hexanediol are completely miscible whereas only 20% of 1,4 butanediol can be dissolved in PTMEG 1000 and less than 10% in PTMEG 2000. PTMEG polyols are hygroscopic and can absorb 2% moisture in an unprotected environment. Gross amounts of water are removed by azeotropic distillation with toluene, and further reduction can be achieved by heating for several hours at 120-150oC under reduced pressure (less than 20mm Hg). PTMEG's are stabilized with antioxidants to prevent degradation during storage and normal handling. However, prolonged heating in the presence of air at 50-60°C will result in partial oxidation and degradation, and thermal decomposition will occur, in absence of air conditions at 210-220°C.
Table 1.10 - Characteristics of commercial PTMEG's|
Molecular weight
|
OH
number (mg de KOH/g)
|
Solidification point (°C)
|
Viscosity (mPas) at 75°C
|
|
650 |
173 |
25 |
55 |
|
1000 |
112 |
26 |
79 |
|
2000 |
56 |
35 |
350 |
1.3.4 - Castor oil based polyols
Castor oil (ricinus oil) is a pale yellow and viscous liquid (gardner viscosity U-V to 25°C), derived from the bean of the castor plant (ricinus communis) that occurs in all tropical and subtropical regions. It is triglyceride of fatty acids that contains 87-90% of ricinoleic acid (cis-12-hydroxyoctadec-9-enoic acid), with a hydroxyl number of 163 mg KOH/g, and average functionality of about 2.7 (Figure 1.31). Castor oil and its derivatives are used as polyols for the PU preparation mainly and in coatings, adhesives, and casting compounds with excellent hydrolytic stability, shock absorbing and electrical insulation properties. They also have been found to be very useful in the preparation of flexible, semi-rigid and rigid PU foams, resistant to moisture, sock absorbing, and with low temperature flexibility. The products with high purity are the recommended for PU's applications.
|
|
Figure 1.31 - Castor oil polyol
Transesterification of castor oil with polyhydroxylated compounds like glycerin trimethylolpropane, or propylene glycol results in polyols with higher or lower functionality. Transesterification with glycerin forms a trifunctional polyol mixture of mono and diglycerides (Figure 1.32), with OH number of 300 mg KOH/g. Castor oil based polyols with OH number of 310 mg KOH/g are used to promote pentanes blowing agent solubility, in rigid foams systems, with good thermal dimensional stability.
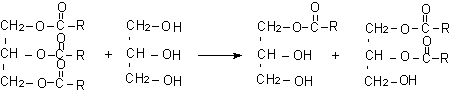
Figure 1.32 - Transesterification of castor oil with glycerin
1.3.5 - Hydroxyl terminated polybutadienes (HTPB's)
Several polyols with hydrocarbon structure are found in the marketplace. The main advantage of the PU based hydrocarbon polyols is the high resistance to hydrolysis, acids and bases. PU's based saturated hydrocarbon polyols have high temperature stability and are used in automotive electronic encapsulation. An important HTPB is obtained by free radical polymerization of butadiene, initiated by hydrogen peroxide and an alcohol as diluents (Figure 1.32).
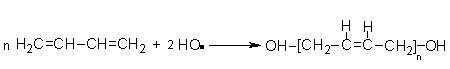
Figure 1.32 - HTPB obtaining reaction
Due
to the free radicals process it has ramifications in polymeric chain, and functionality
slightly higher than two (2.1
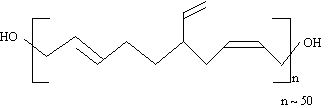
Figure 1.33 - HTPB microstructure
Another type of HTPB is obtained by anionic polymerization of butadiene initiated by sodium naphthalene, and terminated by reaction with ethylene or propylene oxides, following by hydrolysis, resulting in the formation of groups OH primary or secondary, respectively. This commercial HTPB's have functionality 2.0, molecular weights between 2000 and 5000, and usually possess secondary hydroxyl groups. They present microstructure with high quantity of 1,2-vinyl double bonds, which turns them extremely viscous (waxy) at room temperature. Due to the 2.0 functionality, these polybutadiene diols can be used in the thermoplastic elastomers (TPUs) (Chapter 6.3), with excellent hydrolysis and chemical stability, and insulating properties.